The Covid-19 pandemic has resulted in many changes in the way we all work, as well as the drastic health consequences we have been unfortunate to experience or witness. In this short video, our MD, Dr. Seema Sharma describes some business challenges that have presented to us as an agency focussed on the scientific field, and how we have adapted our life science marketing services.
Covid-19
Recent Covid-19
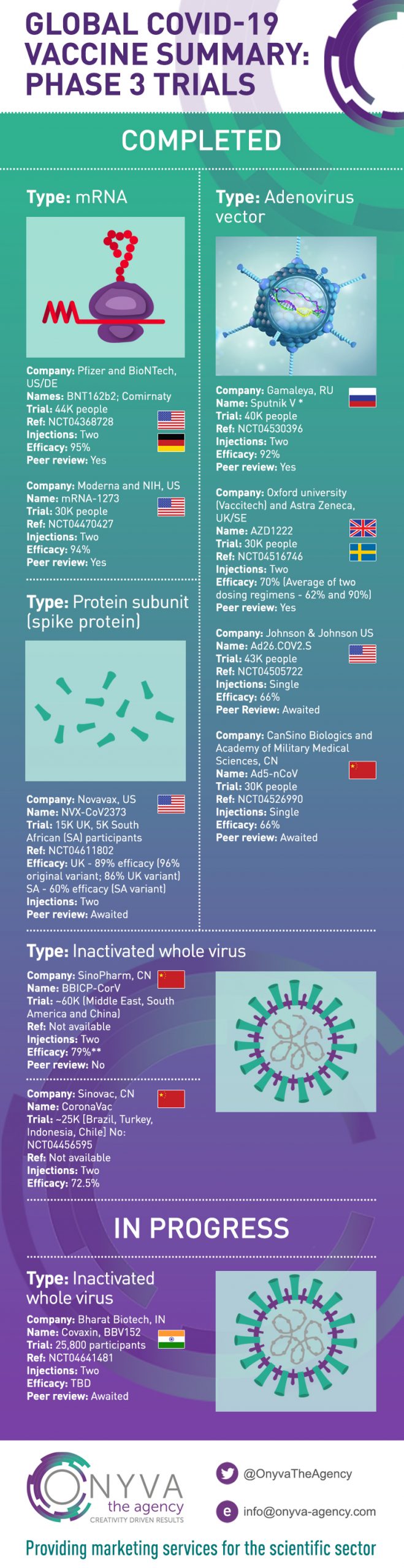
Global Covid-19 Vaccine Summary and Infographic: Phase 3 Trials
Covid-19 Vaccines: Global landscape of Phase 3 Trials
Our vaccine infographic highlights the key Covid-19 vaccines in full or early use, globally. Links to the original reference, where the Phase 3 trial has been published and referenced are shown below.
Sign up to get a free PDF version of our covid-19 vaccine summary infographic
Peer Review: Phase 3 vaccine trials
Type: mRNA
Company: Pfizer and BioNTech – US/DE
Name: Comirnaty, BNT162b2 (Pfizer vaccine)
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
Company: Moderna and NIH, US
Name: mRNA-1273
Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
Type: Adenovirus vector
Company: Gamaleya, RU
Name: Sputnik V
Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia [published online ahead of print, 2021 Feb 2]. Lancet. 2021;S0140-6736(21)00234-8. doi:10.1016/S0140-6736(21)00234-8
Company: Oxford university (Vaccitech) and Astra Zeneca, UK/SE
Name: AZD1222
Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK [published correction appears in Lancet. 2021 Jan 9;397(10269):98]. Lancet. 2021;397(10269):99-111. doi:10.1016/S0140-6736(20)32661-1
For further information, our in-depth post on the results of the AZD1222 vaccine trial, covers more detail.
Peer review awaited: Phase 3 vaccine trials
The following vaccines are awaiting peer-review, so alternative sources for efficacy figures, some of which are press releases of interim analysis from the manufacturer, are shown.
Type: Adenovirus vector
Company: Johnson & Johnson, US
Name: Ad26.COV2.S
Company press release: Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial
Company: CanSino Biologics and Academy of Military Medical Sciences, CN
Name: Ad5-nCoV, Convidecia
Company press release: NMPA Accepts the Application for Conditional Marketing Authorization of CanSinoBIO’s COVID-19 Vaccine, Convidecia TM
Type: Protein subunit (spike protein)
Company: Novavax, US
Name: NVX-CoV2373
Company Press Release: Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial
Type: Inactivated whole virus
Company: Bharat Biotech, IN
Name: Covaxin, BBV152
Company press release: COVAXIN® – India’s First Indigenous COVID-19 Vaccine
Company: Sinopharm, CN
Name: BBIBP-CorV, Sinopharm CNBG’s Covid-19 vaccine
Company press release: China grants conditional market approval for Sinopharm CNBG’s COVID-19 Vaccine
Company: Sinovac, CN
Name: CoronaVac
Company press release: Sinovac Announces Phase III Results of Its COVID-19 Vaccine
* Sputnik V consists of two adenovirus vector constituents (rAd5 and rAd26),
** This figure varies dependent on the source and trials in Brazil (50%), UAE (86%) and from the manufacturer (79%) state differing efficacies.
Publisher: About Us – Onyva The Agency
We are a scientific marketing agency. We have continued to support clients through the current pandemic, producing technical literature, articles and digital marketing support relating specifically to COVID-19 for the medical, healthcare and biotechnology industries. Our scientifically trained team are able to combine up-to-date knowledge on the evolving pandemic, with decades of marketing experience to meet you needs.Take a look at our services.
Get in touch for marketing support: info@onyva-agency.com
COVID-19 Oxford Vaccine Trial Results: Summary
AZD1222, a COVID-19 vaccine that has just undergone phase 3 trials, was co-developed by the spin-out company, Vaccitech, based at the University of Oxford and AstraZeneca. It uses a replication-deficient chimpanzee viral vector based on an attenuated (weakened) version of a common cold adenovirus. The latter has been modified to include the DNA sequence of the SARS-CoV-2 virus spike protein. As a result, this surface spike protein is produced in the recipients body post-vaccination. It is the most antigenic part of COVID-19, eliciting an antibody response, and priming the immune system to counteract future infections.
Overview of COVID-19 Vaccine Trial (Phase 3) Results:
Vaccine Name: AZD1222
Developers: Astra Zeneca + VacciTech (Oxford University) UK
Vaccine details: Chimpanzee Adenovirus vector (attenuated) + Sars-Cov2 spike protein DNA
Approach: Two dosing regimens (inadvertent – see below for explanation)
statistical significance: p-value <=0.0001
| Dose – Day 1 | Dose – Day 28 | Number of test subjects | Efficacy |
|---|---|---|---|
| Half dose | Full dose | n=2471 | 90% |
| Full dose | Full dose | n=8895 | 62% |
Figures released to the press [1] included a composite average efficacy of 70%. In reality there were effectively two trials, due to the difference in doses. The half dose was actually given in error initially, to 2.7K+ test volunteers. This subgroup who received an erroneous administration, actually produced the most pronounced efficacy (90%). Speculative theories around this suggest, the lower dose may stimulate T cell production of antibodies more effectively. Alternatively, the patients who received the higher dose on day 1 may have experienced a more pronounced reaction to components of the viral vector itself. Thus, the response to the second dose was blunted (62%).
Clearly, more research is needed to establish the cause. Additional data on the age and ethnicity breakdown of the individuals included in the higher efficacy subgroup would also be necessary to ensure the trial group was representative of the population at large and no biases were present. For example, an absence of individuals who were 65+, or from ethnic minority groups in the higher efficacy group, has a potential to skew results.
The researchers stated no severe cases of COVID-19 or hospitalisations were recorded in any patients, where the vaccine proved ineffective.
Note that whilst phase 1/2 trials of the vaccine have been published with peer-review [2], we still await the full results and data of the phase 3 trial.
The Oxford University and AstraZeneca team have also made a commitment to broad and equitable global access to the vaccine. https://www.ox.ac.uk/news/2020-06-05-oxford-university-s-covid-19-vaccine-next-steps-towards-broad-and-equitable-global
Pros & Cons of Adenovirus Vaccines Vs RNA vaccines
Pros
Cons
Update 30th December 2020:
The UK Medicines and Healthcare products Regulatory Agency (MHRA) authorised the emergency supply of COVID-19 Vaccine (AZD1222) for the immunisation of individuals 18 years+. This authorisation recommended the two full dose regimens be given – due to a lack of data for the half dose/ full dose regimen at present. They have recommended that the two identical doses be given with a 4 to 12 week interval.
Publisher: About Us – Onyva The Agency
We are a scientific marketing agency. We have continued to support clients through the current pandemic, producing technical literature, articles and digital marketing support relating specifically to COVID-19 for the medical, healthcare and biotechnology industries. Our scientifically trained team are able to combine up-to-date knowledge on the evolving pandemic, with decades of marketing experience to meet you needs.Take a look at our services.
Get in touch for marketing support: info@onyva-agency.com
References:
1. AstraZeneca Press Release: 23 Nov 2020 https://www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr
2. Folegatti PM, Ewer Kj et al., Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Erratum in: Lancet. 2020 Aug 15;396(10249):466. PMID: 32702298; PMCID: PMC7445431.

Continued marketing support
During the recent challenges, we would like to assure existing and new clients that we will continue to support them with all marketing projects.
We are fortunate to be able to work remotely, and have all the infrastructure in place for our team. We are keeping abreast of,and adhering to all of the government advice issued on Covid-19.
We are infinitely grateful to all those working in the medical and healthcare sector who are at the frontline of keeping everyone well, and don’t have the option of working from home.
Please don’t hesitate to get in touch with any queries you may have directly, and stay well.
