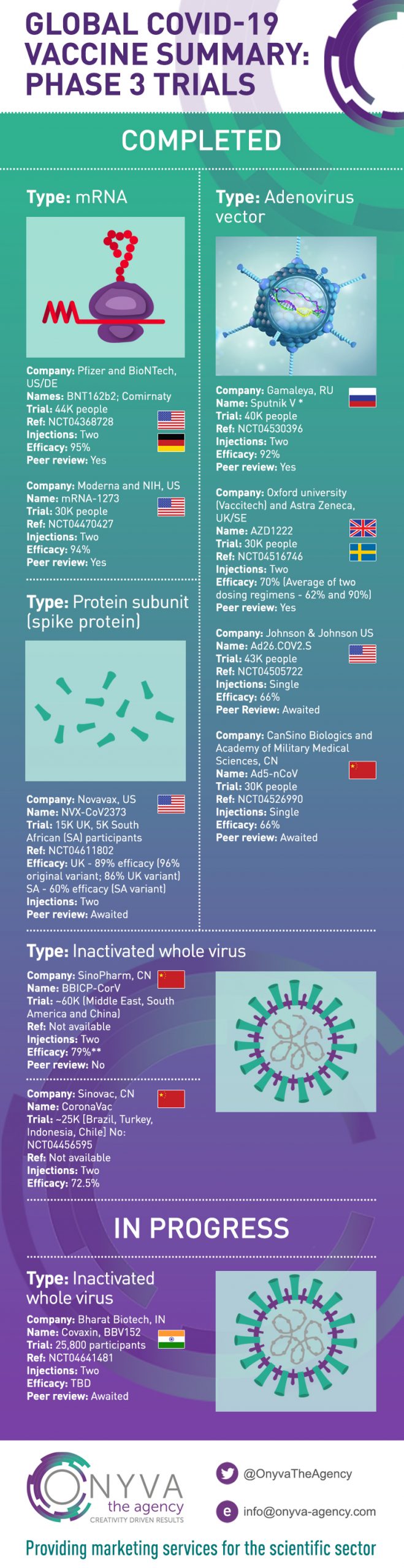
Covid-19 Vaccines: Global landscape of Phase 3 Trials
Our vaccine infographic highlights the key Covid-19 vaccines in full or early use, globally. Links to the original reference, where the Phase 3 trial has been published and referenced are shown below.
Sign up to get a free PDF version of our covid-19 vaccine summary infographic
Peer Review: Phase 3 vaccine trials
Type: mRNA
Company: Pfizer and BioNTech – US/DE
Name: Comirnaty, BNT162b2 (Pfizer vaccine)
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577
Company: Moderna and NIH, US
Name: mRNA-1273
Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389
Type: Adenovirus vector
Company: Gamaleya, RU
Name: Sputnik V
Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia [published online ahead of print, 2021 Feb 2]. Lancet. 2021;S0140-6736(21)00234-8. doi:10.1016/S0140-6736(21)00234-8
Company: Oxford university (Vaccitech) and Astra Zeneca, UK/SE
Name: AZD1222
Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK [published correction appears in Lancet. 2021 Jan 9;397(10269):98]. Lancet. 2021;397(10269):99-111. doi:10.1016/S0140-6736(20)32661-1
For further information, our in-depth post on the results of the AZD1222 vaccine trial, covers more detail.
Peer review awaited: Phase 3 vaccine trials
The following vaccines are awaiting peer-review, so alternative sources for efficacy figures, some of which are press releases of interim analysis from the manufacturer, are shown.
Type: Adenovirus vector
Company: Johnson & Johnson, US
Name: Ad26.COV2.S
Company press release: Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial
Company: CanSino Biologics and Academy of Military Medical Sciences, CN
Name: Ad5-nCoV, Convidecia
Company press release: NMPA Accepts the Application for Conditional Marketing Authorization of CanSinoBIO’s COVID-19 Vaccine, Convidecia TM
Type: Protein subunit (spike protein)
Company: Novavax, US
Name: NVX-CoV2373
Company Press Release: Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial
Type: Inactivated whole virus
Company: Bharat Biotech, IN
Name: Covaxin, BBV152
Company press release: COVAXIN® – India’s First Indigenous COVID-19 Vaccine
Company: Sinopharm, CN
Name: BBIBP-CorV, Sinopharm CNBG’s Covid-19 vaccine
Company press release: China grants conditional market approval for Sinopharm CNBG’s COVID-19 Vaccine
Company: Sinovac, CN
Name: CoronaVac
Company press release: Sinovac Announces Phase III Results of Its COVID-19 Vaccine
* Sputnik V consists of two adenovirus vector constituents (rAd5 and rAd26),
** This figure varies dependent on the source and trials in Brazil (50%), UAE (86%) and from the manufacturer (79%) state differing efficacies.
Publisher: About Us – Onyva The Agency
We are a scientific marketing agency. We have continued to support clients through the current pandemic, producing technical literature, articles and digital marketing support relating specifically to COVID-19 for the medical, healthcare and biotechnology industries. Our scientifically trained team are able to combine up-to-date knowledge on the evolving pandemic, with decades of marketing experience to meet you needs.Take a look at our services.